Keep Politics Out Of The Coronavirus Vaccine Approval Process
Marc H. Morial
President and CEO
National Urban League
Maintaining the American public’s trust in the FDA is vital. If the agency’s credibility is lost because of real or perceived interference, people will not rely on the agency’s safety warnings. Erosion of public trust will leave consumers and patients doubting our recommendations, less likely to enroll in clinical studies or to use FDA-regulated products when they should to maintain or improve their health. This is problematic under normal circumstances but especially if we are to ultimately overcome COVID-19. Protecting the FDA’s independence is essential if we are to do the best possible job of protecting public health and saving lives. -- Senior FDA executives Patrizia Cavazzoni, Peter Marks, Susan Mayne, Judy McMeekin, Jeff Shuren, Steven Solomon, Janet Woodcock and Mitch Zeller
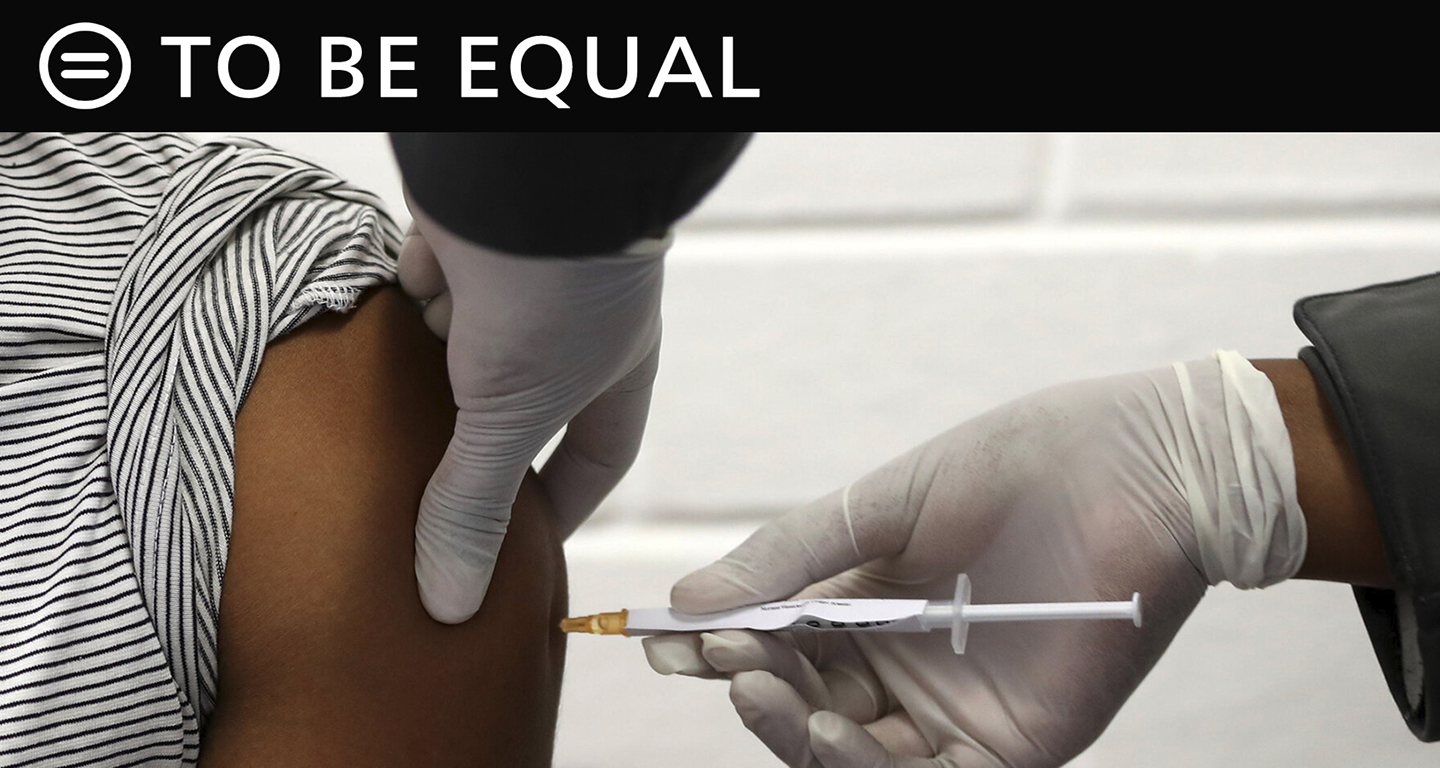
It’s no secret that the White House believes the approval of a vaccine or treatment for COVID-19 would be a boon for the President’s reelection campaign. From the moment the novel coronavirus first reached America’s shores in January, he has regarded it first and foremost as a political inconvenience.
Without evidence, has portrayed anything that delays the “magic bullet” he believes will end the pandemic – or at least appear to end it – as the result of a conspiracy to damage his candidacy. This includes the U.S. Food and Drug Administration approvals process.
If we have any hope of developing a safe, effective vaccine that brings the virus under control, it depends entirely on the FDA’s resolve to resist this shameful political pressure.
In an encouraging move, eight top FDA officials and doctors this week published an op-ed in USA Today pledging that all “decisions will continue to be guided by the best science” and maintain independence from political pressure.
Their statement came a day after executives representing nine companies working on coronavirus vaccines pledged to submit the vaccines for FDA approval only after they’re shown to be safe and effective in large clinical studies.
“We believe this pledge will help ensure public confidence in the rigorous scientific and regulatory process by which COVID-19 vaccines are evaluated and may ultimately be approved.” The pledge was signed by the CEOs of AstraZeneca, BioNTech, GlaxoSmithKline, Johnson & Johnson, Merck, Moderna, Inc., Novavax, Inc., Pfizer Inc., and Sanofi.
It’s worth noting that many of the regulations that still govern the FDA’s drug testing process were developed in the wake of the thalidomide scandal of the early 1960s. The drug, taken by pregnant women, killed thousands of babies in the womb and caused at least 10,000 others in 46 countries to be born with severe deformities. The U.S. escaped this tragedy largely due to the determination of FDA medical officer Frances Oldham Kelsey. “For a critical 19-month period, she fastidiously blocked its approval while drug company officials maligned her as a bureaucratic nitpicker,” the New York Times wrote in a 2015 obituary.
Widespread vaccine use helped eliminate deadly and disabling diseases in the U.S. The last natural outbreak of smallpox – which killed three of every 10 people who contracted it – was in 1949, and the disease was declared eliminated in 1952. Measles was declared eliminated in the U.S. in 2000, although outbreaks among the unvaccinated are triggered by infected travelers bringing the virus from other countries. Rubella, a typically mild illness that can cause serious complications for pregnant women and their babies, was declared eliminated in the U.S. in 2004. Prior to 1955, polio permanently paralyzed thousands of children every year. No cases of polio have originated in the U.S. since 1979.
I have faith that one day, coronavirus can be added to this list. But only if we can maintain faith in our public health institutions. A poll from the Kaiser Family Foundation released this week found that 62 percent of Americans are concerned that a COVID-19 vaccine will be rushed to the market before it's ready because of political pressure from the Trump administration.
A smaller majority, 54 percent, said they wouldn’t take a vaccine that is approved before Election Day.
It’s hard to estimate how much damage the Trump administration has done by allowing politics to override responsible public health policy. The FDA and the pharmaceutical companies must invoke the spirit of Frances Oldham Kelsey and stand as a firewall against his interference.
###
37TBE 9/11/20 ▪ 80 Pine Street ▪ New York, NY 10005 ▪ (212) 558-5300
Connect with the National Urban League
Facebook: https://www.facebook.com/NatUrbanLeague
Twitter: https://twitter.com/naturbanleague
Instagram: https://www.instagram.com/naturbanleague
Website: https://www.NUL.org
Newsletter: http://bit.ly/SubscribeNUL
YouTube: http://bit.ly/YTSubNUL

